A phytoplankton species life cycle comprises four main phases: growth (mitotic and asexual), sexuality (meiotic), quiescence (a sexual or asexual immobile stage with a low metabolic rate which is popularly named cyst) and senescence (population decline and death) (von Dassow and Montresor 2010). Most dinoflagellates have haplontic life cycles, meaning that the vegetative stage (the one undergoing growth) is haploid.

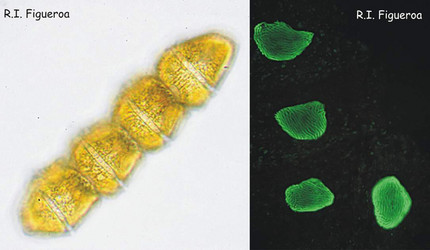
Fig. 1. Vegetative chain of Gymnodinium catenatum (nuclei in the right). The cells divide by mitosis forming chains of up to 64 cells. ©
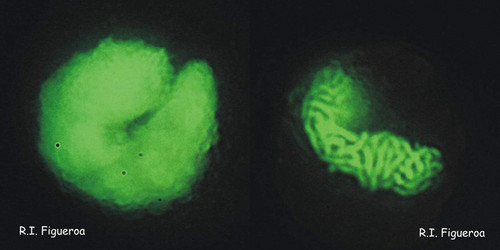
Fig. 2. U-shaped nuclei in vegetative cells of Alexandrium taylori (left) and Alexandrium minutum (right). ©
Asexual reproduction can happen much more quickly, and therefore is the predominant way of reproduction during optimal environmental conditions, but sexual reproduction is essential for species adaptation and survival because it allows for genetic recombination (i.e. genetic variability). During the sexual phase, two haploid cells called gametes fuse to form a diploid mobile zygote (planozygote) that will undergo meiosis to restore the vegetative stage.

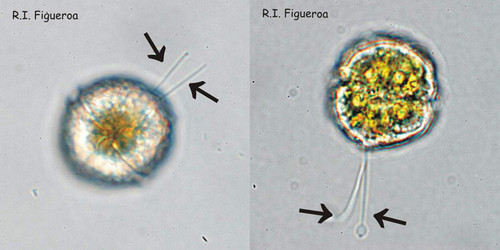
Fig. 3. Examples of planozyotes, which are characterized by two longitudinal flagella (arrows) instead of one, in Alexandrium minutum (left) and Alexandrium taylori (right). ©
The most common pathway reported until very recently was the transition of the planozygote to a quiescent, environmentally resistant stage known as resting cyst (a dormant not motile hypnozygote with a thick wall). Other types of quiescent stages are cysts with a thin wall and less capacity to withstand adverse environmental conditions than the resting cysts. These cysts - found in the bibliography with different names such as temporal, pellicle or ecdysal cysts - can be sexual or asexual, this last case being the fastest way to produce a cyst.

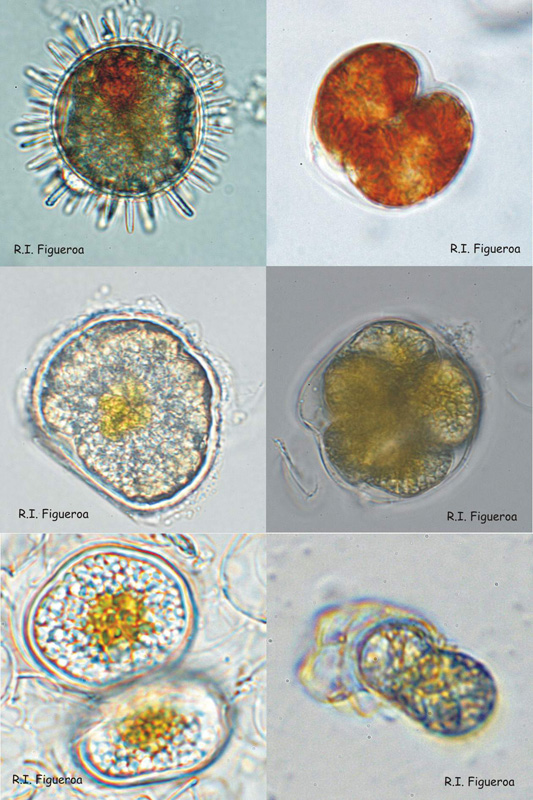
Fig. 4. Examples of resting (sexual) and pellicle (sexual and asexual) cysts.
Top row: Resting sexual (left) and pellicle asexual (right) cysts in Lingulodinium polyedrum.
Middle row: Resting sexual cyst (left) and multiple pellicle also sexual cyst (right) in Alexandrium taylori.
Bottom row: Resting sexual (left) and pellicle asexual (right) cysts in Alexandrium minutum.
©
However, this general pattern is not always followed, and recently reported life cycles have shown to be quite more complex. In some species for which the sexual cycle has been reported no resting cyst is known (e.g. Karlodinium veneficum), and division of the planozygote (meiosis) has been documented in an increasing number of other species; therefore this reinforces the idea that the planozygote can skip cyst formation (e.g. Figueroa and Bravo 2005a, b) and implies that meiosis can occur either after quiescence (resting or pellicle cyst) or without the need of going through a quiescence phase. In some species of dinoflagellates, meiosis (in resting cysts and in planozygotes) has been observed through a phenomenon known as nuclear cyclosis (e.g. von Stosch 1972, 1973, Coats et al. 1984, Barlow and Triemer 1988). This is a process associated with the first meiotic division, which consists of the swirling of the chromosomes within the nuclear envelope (von Stosch 1972).
Other recently discovered pathways in the sexual stage are: (1) in culture gametes can revert to an asexual phase and undergo binary fission (asexual division) rather than fusion (e.g. Gymnodinium nolleri, G. catenatum, Alexandrium taylori or Lingulodinium polyedrium) and (2) planozygotes can undergo meiosis and division without the production of a hypnozygote when they are individually isolated and placed in nutrient replete media, although the sexual process continues if the isolation is to nutrient-deplete media (Figueroa and Bravo, 2005a,b Figueroa et al., 2006a,b). Uchida (2001) did not report planozygote division, but found that planozygotes do not encyst if cell density was below a certain threshold in the species Scrippsiella trochoidea and Gyrodinium instriatum.
But what is causing the shift from an asexual to a sexual cycle? Although the reasons are mainly unknown and need further study, it seems that both endogenous and environmental factors can be responsible for the onset of sexuality, and that each species may have its specific triggering conditions. Sexuality has been traditionally achieved in culture through nutrient depletion, with temperature and light being important modulators of the cyst yield (Sgrosso et al. 2001, Nagai 2004). However, gamete pairing and planozygote formation in nature may not be always linked to nutrient shortage, giving that sexuality has been observed either at the termination of blooms (Persson et al., 2008; Anderson and Wall, 1978) or during active growth (Fukuyo, 2002) or at reaching a cell abundance threshold (Garcés 2004).

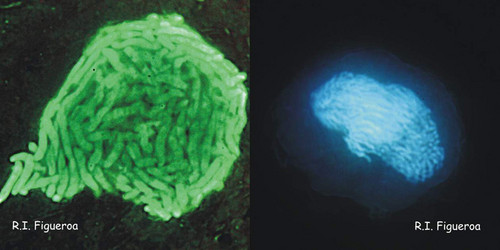
Fig. 5. Planozygote nuclei in Gymnodinium nolleri (left) and Alexandrium minutum (right, undergoing meiosis). ©
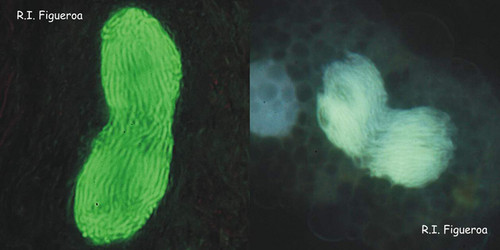
Fig. 6. Mitosis in nuclei of Gymnodinium catenatum (left) and Alexandrium margalefi (right). ©
There are two basic mating systems in dinoflagellates, homothallism and heterothallism, and both groups have isogamous (fusing gametes are similar to each other) and anisogamous (fusing gametes of different sizes) representatives.

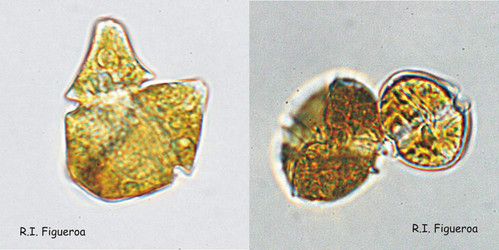
Fig. 7. Isogamous (left, Gymnodinium nolleri) and anisogamous (right, Alexandrium tamutum) gamete pairs. ©
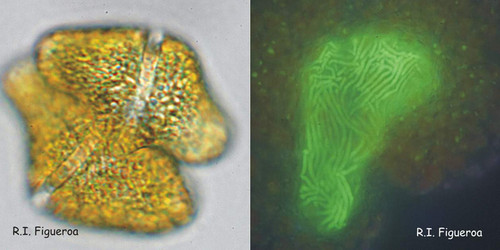
Fig. 8. Fusing gamete pair in Gymnodinium catenatum (left) and its nuclei in fusion process. ©
In homothally, the gametes can be genetically identical. This is the case for example in the species Alexandrium taylori (Giacobbe and Yang 1999). In heterothally, genetically determined factors in the gametes allow successful mating, sexual fusion and meiosis. In this last case, the sexual compatibility can comprise only two different mating types (simple heterothallism), such as in Lingulodinium polyedrum (Figueroa and Bravo 2005b) or more (complex heterothallism), such as in Alexandrium minutum (Figueroa et al. 2007). These definitions when applied to resting cyst (hypnozygote) producing species imply that cyst production and viable progeny are possible within clones of homothallic species (self-fertile), whereas two different clonal strains are needed to obtain viable cysts in heterothallic ones (self-sterile). However, different mating systems can occur within the same species, and for example a continuum between homothally and heterothally has been described in the species Gymnodinium catenatum (Figueroa et al. 2010).
The different sexual and asexual routes can be summarized as shown in the following diagram:

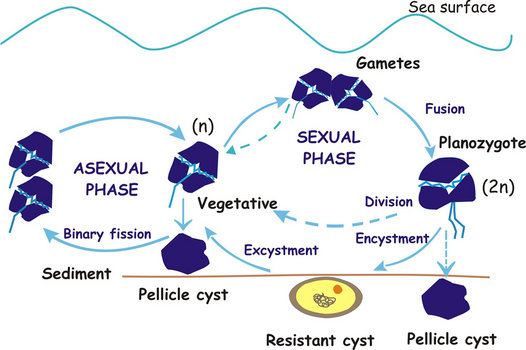
Fig. 9. Dinoflagellate life cycle (modified after Walker et al.1984). ©
At least 10% of all dinoflagellates, and in temperate areas as much as 28%, are resting cyst producers (Persson et al. 2000). Sexual reproduction is thought to be essential for seasonal survival of these species, although asexual resting cysts are also known in Scrippsiella hangoei (Kremp and Parrow 2006). Dinoflagellate cysts in the sediment can provide the inoculum for future blooms after a long time, given that they can remain viable in the sediments up to 100 years (Ribeiro et al. 2010). A heteromorphic life stage such as the cyst can represent an advantage because it allows the allocation of the species into different niches (planktonic and benthic), and because different life stages of the same organism usually interact with the environment in quite different ways.
In many species, planktonic stages are only an ephemeral phase of the organism's life cycle, dispersion and concentration of the dormant non-motile cells being determined by the same forcing functions that control the dynamics of passive particles in the water and in the sediments. Dormancy and maturation of resting cysts are biological processes essential for the dinoflagellate population. The dormancy period is a maturation time in which the biological activity (growth) is suspended. Germination cannot be induced during dormancy, but when it is over, the cyst enters in quiescence, a period in which germination can occur if the surrounding environmental conditions are appropriate.
The length of the dormancy and quiescent periods can affect the timing of blooms. The dormancy can last from hours to days or months and it is species-specific (e.g. 50 days for Alexandrium taylori, 1 month for Alexandrium minutum or only days for Kryptoperidinium foliaceum).
The genetic (endogenous) control of the dormancy has been proved in the species Gymnodinium nolleri (Figueroa et al. 2006c), but there are also exogenous (abiotic and physiological) modulators and internal clocks (endogenous rhythms). For example, a circaannual endogenous clock with an average period of 11 months has been observed under constant environmental conditions for the germination of cysts of the species Alexandrium spp. from eastern Gulf of Maine (Matrai et al. 2005). Germination patterns (continual, sporadic or seasonal) determine each species blooming strategy and the seasonal species succession. Once germination occurs, the size of the cell inoculum, and therefore the chances of becoming a bloom, will be influenced not only by the number of germinating cysts and their dispersion rate, but also by their viability (percentage of survival of the total germinating cells and their ability to perform successful meiosis), which will again depend on several physiological and abiotic factors.


 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site